In the article referred to here (New England Journal of Medicine, requires registration), FDA Chief Makary talks about a new policy that will accelerate drug development.
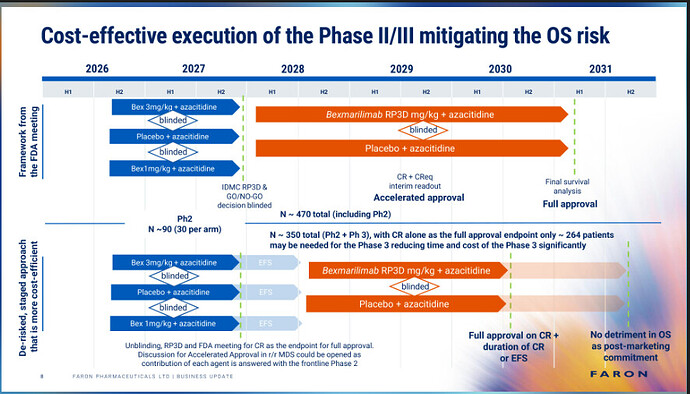
It seems that Faron was already aware of this (from Juho’s latest presentation): the upper chart is the FDA’s “old guidance” and the lower chart describes a new, proposed “De-risked, staged approach,” where survival (OS) would no longer be a hurdle to obtaining marketing authorization.
In other words, surrogate endpoints (for example, improvement in blood values, etc.) are also recognized as proving the drug’s efficacy.
The FDA says that the new guidance will be published later this spring. We’ll see if Faron’s plans will have to be redone once again. Would this be good news for Faron(?); at least it requires a lot of familiarization again.