The highlighted part there again concerns single use, but the problem lies in continuous use:
From the same guideline:
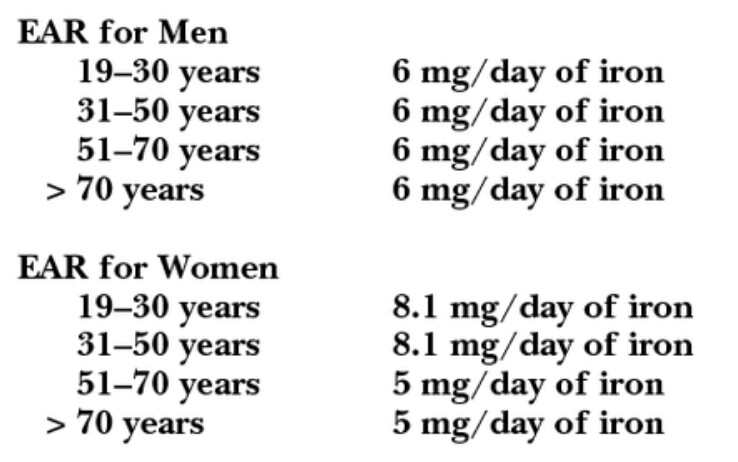
”Hemochromatosis, a disease caused by a mutation in the hemochromatosis (HFE) gene, is associated with an excessive buildup of iron in the body [3,39,99]. About 1 in 10 Whites carry the most common HFE mutation (C282Y), but only 4.4 Whites per 1,000 are homozygous for the mutation and have hemochromatosis [100]. The condition is much less common in other ethnic groups. Without treatment by periodic chelation or phlebotomy, people with hereditary hemochromatosis typically develop signs of iron toxicity by their 30s [3]. These effects can include liver cirrhosis, hepatocellular carcinoma, heart disease, and impaired pancreatic function. The American Association for the Study of Liver Diseases recommends that treatment of hemochromatosis include the avoidance of iron and vitamin C supplements [39].”
In the United States, an estimated one million people suffer from hemochromatosis medlineplus.gov/genetics/condition/hereditary-hemochromatosis/#frequency, meaning iron absorption is not restricted in their intestines, ”Type 1 hemochromatosis is one of the most common genetic disorders in the United States, affecting about 1 million people.” 335 million people live there. If only one in a thousand people in the USA started using Solein (what is Solar Foods’ goal, would it be that little?) that would still be 1000 people among those suffering from the condition. If most of them have already been caught in liver tests or after heart disease has already developed, e.g., 70%, there would still be 300 people left. Let’s further assume that only every second user uses it regularly, i.e., 150 people. Let’s further assume that only every second of them is caught in tests during Solar Foods’ existence and investigations begin into where the iron came from, that’s 75 people. Only every second of them files a lawsuit against Solar Foods with compensation claims, that’s 37 people. Only 18 of them, or half, succeed, and the court finds that the Solein package did not advise testing blood values for latent hemochromatosis before repeated use.
What happens to the company? Lessons can be learned, for example, from the Round Up lawsuits and astronomical compensations, even though the causal link is considerably less clear than with iron.
20-30% of heme iron is absorbed, and 1-10% of non-heme iron, which constitutes 95% of Solein pmc.ncbi.nlm.nih.gov/articles/PMC4179187/ . The company states a figure of less than 5%. However, with heme, it could also be 8%, meaning the company’s estimate is doubled, especially since it’s just a test tube calculation. The protein content is 75%, so a 20g protein bar (27g Solein) could very well contain 2x the company’s estimated 128 mg of absorbable iron = 256 mg/100g. Thus, absorbable iron from the bar, 27x2.56=69 mg, which exceeds the maximum doses for everyone, and especially for those susceptible. That’s almost all so-called excess iron. These absorption matters should rather be exaggerated upwards than perhaps what Solar Foods has done, downwards, because the precautionary principle must be followed in these cases.
The intention is not to disparage a good cause, the bacterium can be changed, but there is a health risk, and a legal risk. Such preparations do not have requirements for extensive testing regarding absorption or side effects, as medicines would. For example, a significant amount of unabsorbed iron also results. Thus, surprises may arise/will arise.
There is also a reputational risk regarding the environmental impacts of the nutrient solution mentioned above, as it is marketed with carbon neutrality. Space protein? Or to be given to everyone, because business? Competitors will certainly seize upon these iron and, for example, ammonia issues, if market share is gained and if they are tried to be silenced. Just be transparent with the reliability of calculations and tests, if there’s nothing to hide. The seven calculations on this forum without official correction indicate that it’s as if someone cannot afford to go public.
![]()