According to this theory, shouldn’t the denominator then be something other than exactly the baseline TD count? For example, if all those 4 had been baseline TI patients who temporarily transition to TD and then back to TI, the number should then be 4/11.
A stock exchange release does not inform the scientific community, but rather communicates scientific matters in an understandable way to the general public, when they are communicated.
An interesting perspective on the matter is also what Faron would achieve with a sloppy press release practice; not a single potential partner in those deal negotiations can be misled, even in theory.
I understand your point, but based on the poster, I don’t see this as quite so problematic.
The TD-TI conversion is presented in the poster in a way that is consistent with baseline TD numbers (4/7 in the first line and 3/23 in the r/r group). Although the text does not explicitly state “from baseline”, the denominators exactly match the numbers of baseline TD patients, and there is no separate “on-treatment TD” group in the poster to which these conversions could refer.
Also, 17% vs 57% does not require explanation within the poster, because 57% refers to a small subgroup of first-line baseline TD, and the TD-TI for the entire cohort is clearly lower. These are different populations, not contradictory figures.
Swimlane diagrams are not, to my understanding, comprehensive transfusion logs. Baseline TD is defined in the IWG criteria based on the preceding period, and it does not require that the patient receives transfusions precisely between study enrollment and treatment initiation. Therefore, in my opinion, the swimlanes also do not invalidate the baseline TD interpretation.
In my opinion, the poster’s data is consistent. However, I agree that the issue lies in how the results have been communicated in the press release, not that the poster itself is contradictory or that 57% is based on the wrong patient population.
Exactly. That’s creative reporting. Believe it or not.
The only possible explanation, benefit of the doubt: both points 1 and 2 must be true
1)the poster represents a later data reading and it has been left unmentioned
2)on the swimlane, Baseline TD patients, very strangely, do not receive blood transfusions between Day 0 and the start of treatment OR these blood transfusions have been left unmarked
In the poster, TI is defined line-by-line, and calculated that way, 65% is technically correct. However, because this is not elaborated in the press release, an overly optimistic picture is inevitably created. As you yourself wrote, “65% maintained TI” and the fact that only about 40% of patients who were completely independent at the start of treatment actually remained without any transfusions are practically two different things. = The true “complete transfusion independence” is closer to 40%.
So, it’s not about incorrect data, but rather that the term is used too loosely in the press release. With a little extra explanation, this could have been easily avoided.
I’m not entirely sure what the correct way to communicate these is. Regulators, researchers, and hematologists certainly understand the TI definition, but it’s not clear to the general public reading a stock exchange release.
Beksmarilimabi** supports bone marrow recovery**
In a study of previously untreated HR-MDS, 57% of baseline transfusion-dependent (TD) patients achieved transfusion independence (TI). In the entire HR-MDS BEXMAB study population, 23% of transfusion-dependent patients achieved independence. The durability of TI was robust, with 65% of patients participating in the study remaining transfusion-independent throughout the treatment. Analysis of bone marrow samples confirmed that the treatment increases the number of red blood cell, platelet, and leukocyte stem cells, providing biological validation for the clinical improvement in blood counts.
Faron’s Chief Medical Officer, Dr. Petri Bono, added: “The new data on transfusion independence are clinically highly significant. For MDS patients, freedom from regular transfusions is a significant determinant of quality of life. By demonstrating that we can make more than half of frontline transfusion-dependent patients independent and at the same time promote deep molecular remissions in high-risk genetic subgroups, we confirm that beksmarilimabi acts on cancer cells and actively rehabilitates the bone marrow environment, which is a key differentiator compared to classic toxic drugs whose sole purpose is to kill cancer cells.”
The combination continues to have an excellent safety profile. In the HR-MDS cohort, no Grade 5 adverse events related to beksmarilimabi use were observed. The encouraging safety profile and clear efficacy signals support the progression of beksmarilimabi to a randomized Phase 3 registration study, addressing the urgent unmet medical need for HR-MDS patients.
I don’t know what the correct reporting method is, but I’m still not putting notes on the side.![]()
It feels like everything here revolves around one person. One must remember that the first payday is partnering, even though it will still take about a couple of years until the sales permit!
I also looked at this, and yes, TI generally refers to red blood cell TI. Here, platelet TI has also been included in the total percentage, without it being specifically mentioned. That is also misleading, although I don’t consider it as serious an error as that baseline TD-TI conversion.
Well yes, this is just because I don’t understand the text I’m reading. Luckily, you wise ones are blessed with the gifts of understanding.
How many presumed PhDs and how much time were needed to understand a single stock exchange release. If the stock exchange release were consumer marketing, this would reek of misleading information (which is at least reprehensible). There are probably also separate rules related to the marketing of investment products (perhaps also applies to stocks). Someone else can figure that out. The burden of proof / burden of explanation will now shift to Faron. Props to Clark for persistence and to Pat for the investigative (and innovative) detective work. It was not a pleasant day for a layman to follow this episode from the sidelines.
It would probably be better if the scientific aspect were not reported at all, and publications were only referred to laconically. Because complex scientific matters simply cannot be explained.
As a layman, I don’t even think about these things that closely, as they are not essential from an investment case perspective; they are just background niche science.
For me, the biggest uncertainty regarding Bex is the highly unpredictably varying research results. At ASH, an attempt was made to highlight the high CR responses in TP53 mutants in the first-line, which can be explained at least in part by patients who received a transplant. overshadowed by these results in the presentation were the rather ordinary results for non-mutant patients in the first-line.
For r/r patients, development has also been quite modest over the last year. At ASH 2024, results for 20 patients were presented:
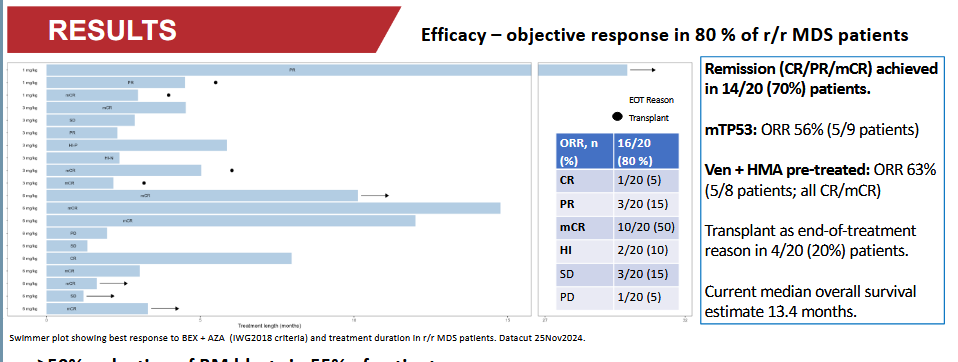
After that, 12 more patients were enrolled in the study, and their responses can be calculated from Esmo’s slides:

The ORR for the first 20 patients was 80%, and for the next 12 patients, the ORR was 33%.
The CR + mCR + PR responses for the first 20 patients were 70%, for the next 12 patients a maximum of 25%. NOTE! at least one patient who achieved a PR response has improved from the 2024 situation.
Of the first 20 patients, 20% received no response, for the next 12 patients, the figure was 67%.
This (either) is not a catastrophe in my opinion, but with these patient numbers, there is a huge amount of variation in the results. Monitoring things is not made any easier by Faron’s very sparse communication, and partly because of this, the air is filled with all kinds of speculation and interpretation of Juho’s eyebrow position.
Another observation from the results. The first 20 were reported according to IWG2018 and the supplementary 12 (also including the previous 20) according to IWG2006. IWG 2018 is clearly stricter than IWG 2006, especially regarding hematological improvement (HI) and transfusion responses. Therefore, the results are not directly comparable. Furthermore, it’s peculiar that the results were better with stricter criteria.
EDIT: Let’s add here that out of the first 20 patients, 9 had TP53, and in the next 12, only 4. Perhaps the distribution of patients by wt/mt doesn’t matter.
Those slides seem to have different IWG criteria. Have these been taken into account in that calculation? I can’t quite keep track of them manually.