Do you really mean that medicine is not what brings added value to investors? Is the value of a drug developer not based on drugs?
Where does the information that “big pharmas don’t believe in this enough” come from?
Do you really mean that medicine is not what brings added value to investors? Is the value of a drug developer not based on drugs?
Where does the information that “big pharmas don’t believe in this enough” come from?
However, medical data is what determines the fate of this venture, whether the results are convertible into money in the partner’s opinion.
Regarding the results:
”In treatment-naïve HR-MDS, 57% of patients who were transfusion-dependent (TD) at baseline successfully achieved transfusion independence (TI).”
In assessing its significance, it should be taken into account that the group in question had a particularly high number of TP53 mutants, which shortens responses and lifespan, at least without Bex. For TI, there must be an improvement in blood values for a certain period. Thus, a direct comparison to first-line treatment in other trials cannot be made. I haven’t yet found information on what the TD and TI proportions have historically been for these mutants. Patient numbers are small, so phase 3 is awaited again.
And how on earth do you know that? Or do you have such close contacts with every big pharma that they have told you that we really don’t believe in this?
Making deals takes time. Especially when we outsiders don’t know at all what details are being discussed, whether it’s blood cancers or mTp53-associated different entities or something else entirely? Yes, there are very strict NDAs throughout the entire process, until either a deal is reached or not. And even after that, of course.
The mTp53 indication precisely reinforces Faron’s message that efforts are being made to understand what kind of cancer it is, and how different types of cancers should be treated. And the results seen now provide strong indication that they are on the right track.
As a reminder, there are also over 20 years of research behind this. It is not advisable to set limits on sharing research data if it relates to Faron and Bex’s PoC. And regarding the PoC, the puzzle pieces seem to be increasingly falling into place. For those interested, I still recommend this recent presentation by Maija on where it all started and how we have now arrived at this point: https://youtu.be/WXq66K2-iQQ?si=0e8kpCUA3vwOA-oW
Me, here, today and now: From an investor’s perspective, data is the best possible, and from this, there is still a long way to practical patient work.
Not to mention what kind of diseases and treatments are being discussed — and with whose medicines and collaboration.
Quite a matrix in the fight against cancer. What is your expected value as a faron investor?
Here’s an opportunity to catch a big fish with nets long-term, or a smaller catch with a single line. I myself advocate for maximum value appreciation and the realization of added value for investors and owners.
What concerns me is the wt tp53 CR rate, 20%. Is there reason to worry about how BEX performs in these? On the other hand, 4 MRD- cases, which indicates strong responses. And combining with the now published Esmo data, there would be, in addition to two CRs, 4-5 mCR responses. So if n is 10 in both groups (1 wt → mTP53 after Esmo), the CR+mCR would still be 60%…
Bex studies are also cutting-edge in this respect because they involve CR/cCR responses, not mCR, and therefore the results are genuinely OS-relevant.
This LinkedIn post by Bono, linked above, regarding the Key highlights, is a good example of Faron’s very vague way of handling investor communications:
\u003e Duration of CR in treatment-naive/frontline pts: 12.1 months
Faron published a stock exchange release this morning concerning matters presented at ASH, and now Bono is posting information that cannot be found in the stock exchange release or ASH slides. The question arises for me, why isn’t this information in the stock exchange release, and instead Bono is sending such information to LinkedIn?
My assumption is that the reported 12.1-month duration of response does not pass any scientific scrutiny, and therefore it could not be announced in the stock exchange release. A somewhat similar case was when we read from Carnegie’s update that Faron was preparing to proceed with a CRO to the next phase of the study.
Well, that could be the duration of the longest response, how can we know? More detailed information about such a highlight should also be found in the stock exchange release so that it could be evaluated in any way.
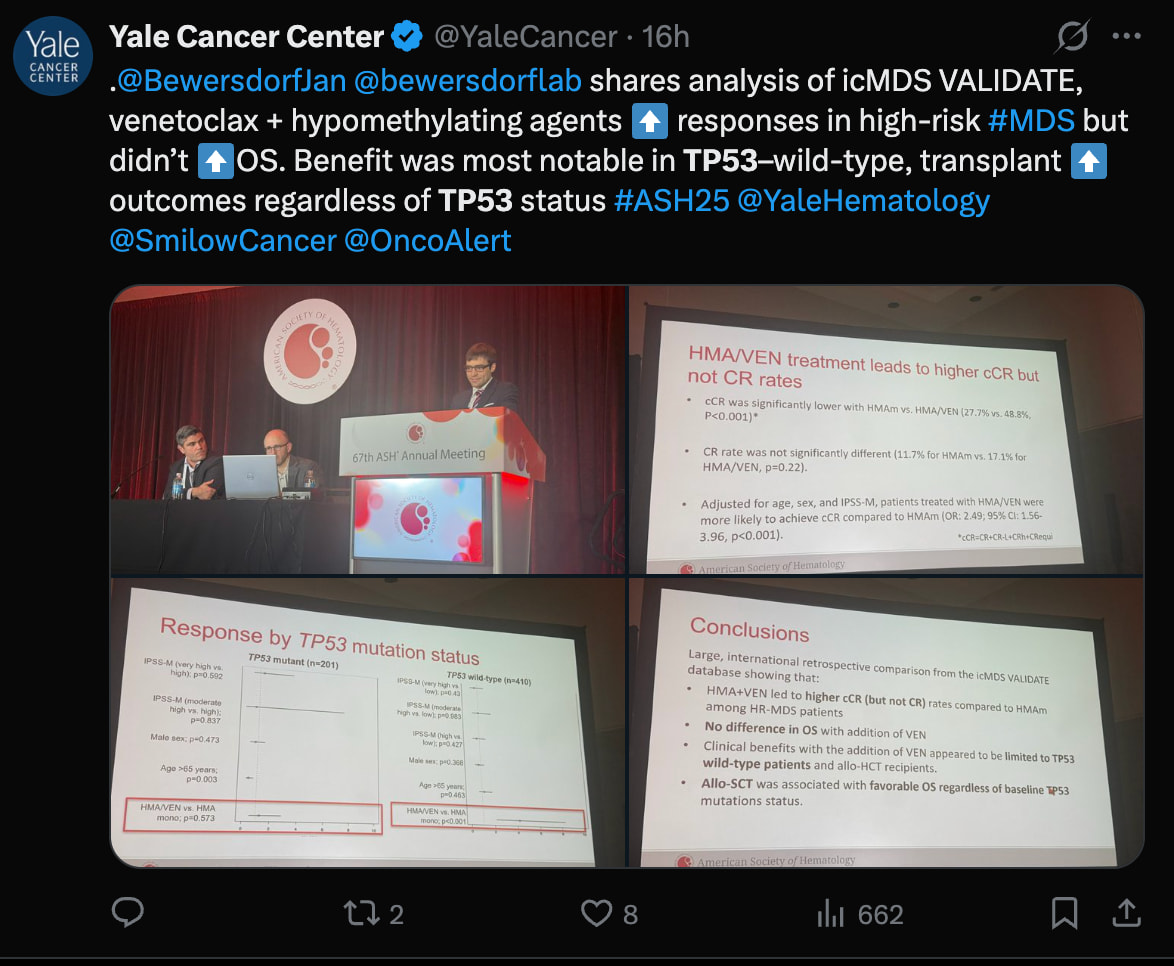
https://meetings-api.hematology.org/api/abstract/vmpreview/291083
HMA+VEN increases cCR, but not CR or OS. No benefit in TP53 mutation. More pressure on Big Pharma to find an effective myeloid immunotherapy.
Bex produces deep and durable responses precisely in the TP53-mut population, where VEN completely fails.
So, the chief medical officer posts under their own name and face figures that would not withstand any scientific scrutiny?
Well, the studies are ongoing, the figures are based on current information, but it’s very difficult to imagine Bono babbling about figures without a solid scientific basis.
The responses of TP53 mutants reported at ASH in the first line were quite exceptional, as 7/8 of those who responded had a reported CR. That is, out of ten patients, 8 received at least some response, and for 7 of them, it was CR. Among Wt TP53 patients, 9 responded, of which 2 were CR.
This might shed some light on how this is possible:
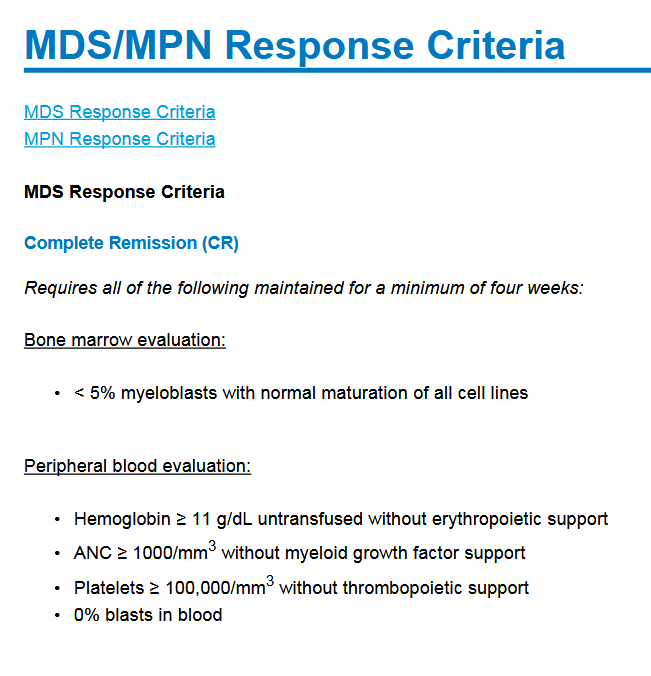
And then an important exception to the matter:

In practice, the CR response of patients receiving a transplant does not need to last longer than the time of sampling for them to be reported as CR patients. Otherwise, the response should last at least 4 weeks for all criteria. Of those 7 CR patients, 5 had received a transplant, which likely explains a large part of these “incredible” responses in mTP53 patients.
The source has over 25 abstracts at ASH:
Commute day to Turku again.
This time, Faron’s floor is fully lit.
Work is being done, a pleasant sight for the owner.
(delete if you want, but I thought it appropriate to provide the information so that the “electricity bills overdue” status from a couple of weeks ago doesn’t remain in effect ![]() )
)
Proactive’s normal simplified version (in my opinion, suitable for informing a non-medical audience)
Bono’s post. So, this is about the duration of CR (Complete Response). I wouldn’t be able to doubt its validity on any grounds, my bad. Why wasn’t it in the stock exchange release? Perhaps it wasn’t considered such significant information by anyone other than Bono.
The CRO (Contract Research Organization) matter comes up again. Faron has stated in a few stock exchange releases that it is preparing for Phase 3. “We will now focus on conducting the registrational Phase 2/3 trial for bexmarilimab in treatment-naïve patients with HR-MDS” In September, they publicly sought employees for, among other things, CRO collaboration. In the November investor presentation https://faron.com/wp-content/uploads/2025/11/Faron-non-confidential-corporate-deck-November-2025.pdf
it states “2025: CRO & key supplier selections for Phase
II/III BEXMAB-2 trial.”
Large trials cannot be conducted without a CRO.
IWG2023, which is used now and in the future, although Faron has also reported some responses according to IWG2006 in the summer?, (edit and now Phase 1/2 results) no longer has a 4-week limit, whereas 2006 does. A response is a response even if the patient dies the next day. Or if they went for a transplant, in which case the DoR (Duration of Response) would be 1 day, but these are likely censored in these non-randomized studies. OS (Overall Survival) is indeed continued.
In BEXMAB, bone marrow samples were taken every 3 months during longer follow-up, and blood tests apparently once a month. That also presents a source of inaccuracy. Is DoR considered to end on the same day when blasts attacked again, or 3/2, i.e., 1.5 months ago? Thus, when evaluating with IWG2023, DoR should be considered. If CR is long, good. If CR is a one-time measurement leading to transplant, good. A transplant guarantees at least as good OS as a long CR.
Edit. Faron does indeed report results according to IWG2006, or a small change came in 2018, because that criteria set was in use for Phase 1/2. Even if it is now known that responses according to IWG2023 would be more significant.
Well, uhm, IWG2006 was, however, at least according to the slides, in use when the results were reported:
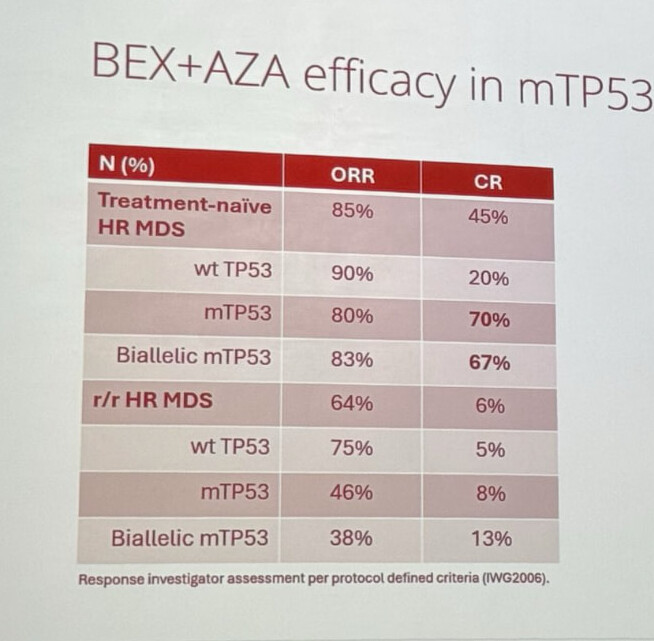
True. It raises ORR because bone marrow clearance is calculated even without blood cell responses, and it can lower CR because the red blood cell response, i.e., Hb, is stricter.
The investor presentation and webcast are announcements made for investors. Should it have been mentioned separately in the company announcement? Perhaps it wouldn’t have hurt, at least. I myself understood it to be part of the preparation. If the partnering (in spe) happens later, a delay in Phase 3 is certain, if the organization is not already ready.
CR data is inflated because a significant portion of patients have progressed to transplant. A large proportion is explained by the fact that the study includes healthier patients who can tolerate transplant treatment, and they are treated in a highly specialized center. Additionally, the number of TP53 mutations is high; transplant treatment is desired for these patients. When proceeding to transplant, there is no waiting to see what happens, whether the response lasts or not; instead, it is carried out if the patient is eligible and a transplant is available.
That CR data would have absolutely required at least a swimmer plot alongside it, which would show the time to response, duration, and the start date of progression to transplant treatment.
Regarding the duration of responses. IWG2023 does not, in my opinion, explicitly state what duration of response is officially accepted. However, we know that short-duration responses do not correlate with OS. Especially in TP53. The FDA certainly knows this and can inquire about the duration of responses if necessary.
The now reported duration of responses in mTP53 patients (10.2 months) was at least omitted from the stock exchange release, because it was a KM estimate, based on 8 patients of whom 6 were censored, the confidence interval was 0.3 months - NR! So the data, although “true and correct” in itself, is just noise, because only 2 events (loss of response) have been documented! And note that patients who received a transplant can no longer lose response in that study; instead, they are censored and exit the at-risk population when transplant treatment begins.
In wt patients, the KM estimate for the duration of responses was 6.2 months; here, significantly fewer were censored, 2/9; this is a much more reliable figure. (1.9 months - NR)
I don’t know where Bono’s 12 months comes from; perhaps it’s an individual patient’s result, like a data highlight / case report. One can write about such things on LinkedIn if desired; it doesn’t seem to be information relevant enough to affect the company.
Now that CK data is out, apparently all that’s coming, what is your overall assessment - will this data lead to the next phase trial (let the number be 2 or 3, it’s all the same to me) or does Bex’s story end here?
I used to think that if bex had been in Big Pharma’s pipeline, it would have been discarded already after MATINS. But now it’s a different situation, Faron’s practically only asset. The sunk cost fallacy is too strong a force; funding will surely be found from somewhere to continue. Even in small steps. It’s very interesting to see how Faron will proceed, because if that efficacy in TP53 patients is truly believed, the drug should be studied solely in this subgroup. Would there be a place for the separate Phase 2 I’ve been longing for? Now there was only talk of stratification (meaning ensuring the groups are balanced in this regard), which doesn’t change the big picture yet. Let’s remember again that VERONA failed, but it was stated post hoc (information on these in ASH abstracts) that it would have succeeded, perhaps, if it had been studied in younger / ASXL1-positive / excess blasts patients / or only in specialized centers.
I had already set certain limits for myself earlier, and as a result, I decided today to sell half of my Faron position. I don’t have to waste so much of my life on this stock anymore, as it’s no longer overweight in my portfolio. Let’s see if, with my luck, partner news comes out within a couple of days.
However, I didn’t follow my mentor’s advice: “if the position pisses you off for even a moment, sell everything”